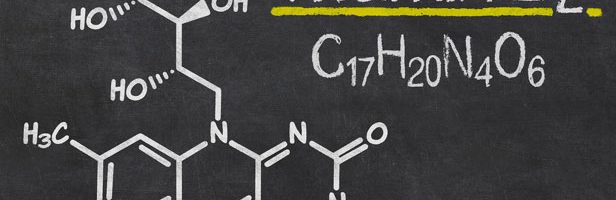
The name “riboflavin” comes from “ribose” (the sugar whose reduced form, ribitol, forms part of its structure) and “flavin”, the moiety which imparts the yellow color to the oxidized molecule (from Latin flavus, “yellow”).
Riboflavin is essential for the intermediary metabolism of carbohydrates, amino acids, and lipids, and also supports cellular antioxidant protection. The vitamin performs these functions as coenzymes (i.e., flavoenzymes)-Flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). Because of these fundamental roles of riboflavin in metabolism, a deficiency of the vitamin first manifests itself in tissues with rapid cellular turnover, such as skin and epithelium.
Because riboflavin occurs in most foods as protein complexes, the utilization of the vitamin in foods depends on the conversion of these forms to free riboflavin. This occurs in the intestinal lumen. The free form of riboflavin is transported into the intestinal mucosal cell; however, much of that form is quickly converted within the enterocyte to FMN by a flavokinase. Thus, riboflavin enters the portal circulation as both the free vitamin and FMN.
Riboflavin is transported in the plasma as free riboflavin and FMN, both of which are bound in appreciable amounts to plasma proteins such as immunoglobulins (IgA, IgG, and IgM), fibrinogen, albumin.
Riboflavin is transported into cells in its free form. The greatest concentrations of the vitamin are found in the liver, kidney, and heart. Significant amounts of free riboflavin are found only in retina, urine, and cow’s milk, where it is loosely bound to casein. Although the riboflavin content of the brain is not great, the turnover of the vitamin in that tissue is high and the concentration of the vitamin is relatively resistant to gross changes in riboflavin nutriture (1).
After it is taken up by the cell, free riboflavin is converted to its coenzyme forms in two steps, both of which appear to be regulated by thyroid hormones. Riboflavin is rapidly excreted, primarily in the urine. Therefore, dietary needs for the vitamin are determined by its rate of excretion, not metabolism. In a riboflavin-adequate human adult nearly all of a large oral dose of the vitamin will be excreted, with peak concentrations showing in the urine within a couple of hours.
Uncomplicated riboflavin deficiency appears only after three to four months of deprivation of the vitamin. Signs include inflammation around the mouth and oedema of the oral mucosa, seborrheic dermatitis around the nose and mouth and scrotum/vulva, and anaemia. Riboflavin-deficient humans also experience neurological dysfunction involving nerve pain in the extremities as well as decreased sensitivity to touch, temperature, vibration, and position.
Many tissues are affected by riboflavin deficiency. Therefore, deprivation of the vitamin causes loss of appetite, impaired growth, and reduced efficiency of food utilization. These are accompanied by abnormally low activities of a variety of flavoenzymes especially erythrocyte glutathione reductase (EGR) which is critical in resisting oxidative stress in red blood cells. In summary, riboflavin deficiency results in impairments in the metabolism of energy, amino acids, and lipids (2).
Riboflavin deficiency also produces changes in the small intestine which are associated with reduced enteric absorption of dietary iron, resulting in secondary impairments in nutritional iron status in riboflavin-deprived individuals.
Several factors can contribute to riboflavin deficiency: Inadequate diet is the most important cause of riboflavin deficiency. Frequently, this involves the low consumption of milk, which is the most important source of the vitamin available in most diets. In industrialized countries, riboflavin deficiency occurs most frequently among alcoholics, whose dietary practices are often faulty, leading to this and other deficiencies. However, high intakes of alcohol appear to antagonise the utilisation from foods.
Excessive training can also increase riboflavin losses as can some illnesses such as HIV. Although earlier studies purported to show reduced riboflavin status among some women using oral contraceptive agents, more recent critical studies have failed to detect any such interaction. Patients receiving diuretics or undergoing hemodialysis experience enhanced loss of riboflavin (as well as other water-soluble vitamins). It has been estimated that as many as 27% of urban American teenagers of low socioeconomic status have subclinical riboflavin deficiency (3).
The toxicity of riboflavin is very low, and thus problems of hypervitaminosis are not expected. Probably because it is not well absorbed, high oral doses of riboflavin are essentially non-toxic.
Sources of Riboflavin
Riboflavin is widely distributed in foods, where it is present almost exclusively bound to proteins. Rapidly growing, green, leafy vegetables are rich in the vitamin; however, meats and dairy products are the most important contributors of riboflavin to diets, with milk products providing about one-half of the total intake of the vitamin.
Riboflavin is stable to heat; therefore, most means of heat sterilization, canning, and cooking do not affect the riboflavin contents of foods. However, exposure to light (e.g., sun-drying, sunlight exposure of milk in glass bottles, cooking in an open pot) can result in substantial losses, as the vitamin is very sensitive to destruction by light. Thus, exposure of milk in glass bottles to sunlight can result in the destruction of more than half of its riboflavin within a day. This can be exacerbated by sodium bicarbonate, which is used to preserve vegetable colours. Also, because riboflavin is water soluble, it leaches into water used in cooking and into the drippings of meats. As riboflavin in cereal grains is located primarily in the germ and bran, the milling of such materials, which removes those tissues, results in considerable losses in their content of the vitamin. For example, about half of the riboflavin in whole-grain rice, and more than a third of riboflavin in whole wheat, is lost when these grains are milled. Parboiled (“converted”) rice contains most of the riboflavin of the parent grain, as the steam processing of whole brown rice before milling this product drives vitamins originally present in the germ and aleurone layers into the endosperm, where they are retained.
The non bound forms of riboflavin in foods, and free riboflavin, appear to be well absorbed. In contrast, flavin complexes, such as those found in plant tissues, are stable to digestion, and thus un-available. In general, riboflavin in animal products tends to have a greater bioavailability than that in plant products.
FUNCTIONS OF RIBOFLAVIN
Riboflavin functions metabolically as the essential component of two coenzymes which act as intermediaries in transfers of electrons in biological oxidation–reduction reactions. The flavoproteins, which are a large group of enzymes involved in biological oxidations and reductions, are essential for the metabolism of carbohydrates, amino acids, and lipids. Some are also essential for the activation of the vitamins pyridoxine and folate to their respective coenzyme forms, others participate in antioxidant protection.
The fundamental metabolic roles played by flavoenzymes give riboflavin relevance to health in various ways. Accordingly, riboflavin status affects responses to pro-oxidants, homocysteine, carcinogenic factors, and malaria. Flavoenzymes participate in protection of erythrocytes (red blood cells) and other cells against oxidative stress.
Cardiovascular Disease
There is evidence that riboflavin may play a role in reducing risk of vascular disease. Dietary riboflavin intake has been found to be inversely correlated with serum homocysteine levels (4). The Framingham Offspring Study found elevated plasma homocysteine levels in subjects with relatively low plasma riboflavin levels (5). Homocysteinemia has been associated with increased risks of vascular disease, total and cardiovascular disease-related mortality, stroke, dementia, Alzheimer’s disease, fracture, and chronic heart failure (6).
Riboflavin may be most important in individuals with a polymorphism of methyltetrahydrofolate reductase (i.e., the heat-sensitive form of the enzyme) that leads to increased risk of vascular disease (7). In vitro studies have shown that riboflavin can stabilize this isoform (8).
Malaria
Riboflavin deficiency appears to protect against malaria, decreasing parasitemia and signs of infection. The metabolic basis of this protection is thought to involve increased vulnerability of erythrocytes (red blood cells) to destructive lipid peroxidation caused by the oxidative stress resulting from the infection (Plasmodium sp.) (10). Hence, they tend to autolyze before the plasmodia they contain can mature, reducing the levels of parasite and decreasing the symptoms of infection. The infected erythrocyte has been found to have an increased need for riboflavin (11. In addition, malarial parasites have been shown to be even more susceptible than erythrocytes to reactive oxygen species. Therefore, it has been suggested that marginal riboflavin deficiency may be preferentially deleterious for both the parasite and the infected cell.
Defects in Fat Metabolism
Riboflavin plays essential roles in lipid metabolism. Accordingly, riboflavin treatment has been found useful in treating cases of recurrent hypoglycemia and lipid storage myopathy in individuals with deficient expression of these flavoenzymes.
Parkinson’s disease
Abnormal riboflavin status in the absence of a dietary deficiency has been detected in Parkinson’s disease (PD), while the classical determinants of homocysteine levels (B6, folic acid, and B12) were usually within normal limits. After one month the riboflavin status of the patients was normalized and all patients who completed 6 months of treatment showed improved motor capacity during the first three months and while most reached a plateau, some continued to improve in the three to six month interval. Their average motor capacity increased from 44 to 71% after 6 months, increasing significantly every month compared with their own pretreatment status. The data show that the proposed treatment improves the clinical condition of PD patients. Riboflavin-sensitive mechanisms involved in PD may include glutathione depletion, cumulative mitochondrial DNA mutations, disturbed mitochondrial protein complexes, and abnormal iron metabolism. More studies are required to identify the mechanisms involved (12).
Alzheimers Disease/dementia
In cross-sectional studies, elevated plasma homocysteine levels have been associated with poor cognition and dementia. An increased plasma homocysteine level is a strong, independent risk factor for the development of dementia and Alzheimer’s disease. It has been suggested a diet high in B vitamins may be protective against dementia, given the beneficial effect in reducing homocysteine levels (13)
- Bates, C.J., 1997. Eur. J. Clin. 51, S38–S42.
- Depeint, F., Bruce, W.R., Shangari, N., et al. 2006. -Biol. Interactions 163, 94–112.
- Powers, H.J., 2003. Am. J. Clin. Nutr. 77, 1352–1360
- Ganji, G. and Kafai, M. R. (2004). J. Clin. Nutr. 80, 1500.
- Jacques, P. F., Bostom, A. G., Williams, R. R., et al. (2002). Nutr. 132, 283.
- Selhub, J. (2006) Nutr. 136, 1726S–1730S.
- Hustad, S., Ueland, P. M., Vollset, S. E., et al. [2000] Chem. 46, 1065.
- McNulty, H., McKinley, M. C., Wilson, B., et al. (2002). J. Clin. Nutr. 76, 436.
- Agte, V. V., Paknikar, K. M., Chiplonkar, S. A., et al. (1998). Biol Trace Elem. Res. 65, 109.
- Das, B. S., et al. (1988). J. Clin. Nutr. 42, 227.
- Dutta, P. (1991). Protozool. 38, 479.
- G. Coimbra ; V.B.C. Junqueira (2003) Brazilian J Med Biol Res,36,1409-1417
- Seshadri S et al (2002) N Engl J Med 346:476-83
Key words-riboflavin, malaria, homocysteine, cardiovascular disease, alzheimers disease/dementia, parkinson’s disease